Faculty Research
All chemistry/biochemistry faculty at IWU are professionally active and are engaged in research with Illinois Wesleyan students. Learning is enhanced at Illinois Wesleyan by the quality, individualized instruction you'll receive from our faculty. Our faculty will work closely with you, in and outside of class, in lab, and in collaborative research. You do not need to be a chemistry major to do research. Please e-mail the faculty directly or stop by their office, should you be in interested in research options. It is advised that you contact faculty a semester prior to when you wish to begin research.
Dr. Brian Brennan - Bioorganic Chemistry
Sickle-cell disease is a genetic blood disorder that is characterized by the presence of sickle-shaped red blood cells in the body. These “sickled” cells clog small capillaries, leading to severe pain and organ damage. The disease is caused by a single point mutation in the gene coding for the -chain of hemoglobin and leads to the incorporation of a valine surface residue in place of glutamic acid.2 Under low oxygen conditions, a binding surface for the valine residue is revealed, allowing for an intramolecular interaction that leads to the polymerization of sickle cell hemoglobin and results in malformation of the cell. Despite the fact that the molecular mechanism of SCD is well understood, no effective treatment exists. Therefore, the development of molecules capable of preventing sickle-cell hemoglobin (HbS) polymerization would be highly desirable. The research in the Brennan laboratory focuses on using modern screening and selection strategies (phage display libraries and one-bead-one-compound combinatorial libraries) towards the discovery of HbS ligands capable of disrupting HbS polymerization.
Brian Brennan Publications:
Bockman, M. R.; Miedema, C. J.; Brennan, B. B. A Discovery-Oriented Approach to Solid-Phase Peptide Synthesis. J. Chem. Educ. 2012, 89, 1470-1473.
Buhrlage, S. J.; Bates, C. A.; Rowe, S. P.; Minter, A. R.; Brennan, B. B.; Majmudar, C. Y.; Wemmer, D. E.; Al-Hashimi, H.; Mapp, A. K. Amphipathic Small Molecules Mimic the Binding Mode and Function of Endogenous Transcription Factors. ACS Chem. Biol. 2009, 4, 335-344.
Rowe, S. P.; Casey, R. J.; Brennan, B. B.; Buhrlage, S. J.; Mapp, A. K. Transcriptional Up-regulation in Cells Mediated by a Small Molecule. J. Am. Chem. Soc. 2007, 129, 10654-10655.
Dr. Tom Kwiatkowski
Kwiatkowski’s lab has two unique areas of research.
Project 1:
The glucose uptake machinery in cells and muscle fibers is a robust and highly regulated system used to import extracellular glucose for intracellular metabolic processing. The majority of glucose uptake in skeletal muscle is done through stimulated exocytosis and fusion of vesicles to the plasma membrane to enrich the cellular exterior with glucose transporter protein GLUT4. In our investigation, we found evidence that GLUT4 containing vesicles (GSV) and the glucose uptake regulators AS160, rab8A, and rab10 serve an additional role in the plasma membrane repair response. My research focuses on targeting glucose uptake regulators to develop new therapies for muscle disease and injury. Through three specific aims, we will test the hypothesis that the myosin-Va motor protein is involved in the exocytotic membrane repair response through its association with rab G proteins 8A and 10.
Aim1: Examine the contribution of myosin-Va in the repair response of two muscle derived cell lines.
Aim2: Establish protein interaction differences with myosin-Va in cells and muscle tissue, with and without damage.
Aim 3: Investigate if stimulating or overexpressing myosin-Va can improve membrane repair in muscle derived cell lines.
Project 2:
The consumption of electronic cigarettes (e-cigs) with a variety of e-liquids is increasing at an alarming rate without thought given to the unrealized health effects. Recent studies find that the use of e-cigs may correlate with an increase in cardiovascular disease, liver disease, renal injury, and the neural stress response. Because e-cig users display pathological defects in multiple organ systems, it is likely that the secretion of compounds and cytokines from lung cells into the pulmonary circulation is exacerbating various health problems. This project will investigate the hypothesis that e-cig vapor induces secreted cytokines from lung epithelial cells that cause a hypertrophic response in cardiomyocyte cell lines.
Aim1: Examine the differences in morphology and inflammatory signal transduction in H292 lung epithelial cells exposed to different quantities of vaporized nicotine, propylene glycol, and glycerin.
Aim2: Determine if the exposure of e-cig induced H292 cytokines upregulate hypertrophic signal transduction pathways in cardiomyocytes.
Aim3: Measure changes in pro-inflammatory markers in lung and heart tissue of mice
Publications
- Thomas A. Kwiatkowski & Aubrey L. Rose, Ana Capati, Rosalie Yan, Diana Hallak , Noah Weisleder “Multiple poloxamers increase plasma membrane repair capacity in muscle and non-muscle cells.“ The American Journal of Physiology-Cell, 2019
- Thomas A. Kwiatkowski & Liubov V. Gushchina, Noah Weisleder. “Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases.“ Pharmacology and Therapeutics, (2017).
- Thomas A. Kwiatkowski, Aubrey Rose, Rachel Jung, Kevin McElhanon, Brian Paleo, Eric X Beck and Noah Weisleder, “Muscle Fibers Utilize its Glucose Uptake Machinery to Reseal Sarcolemma Disruptions & Stimulating Affiliated Rab G Proteins Improves Repair Capacity in Muscle Dystrophic Mice.“ The Journal of Cell Biology, (In preparation)
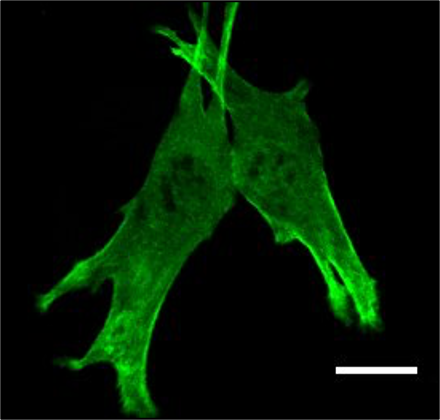
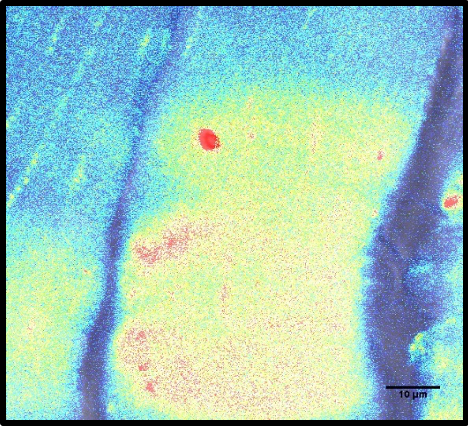
Electroporated muscle fiber that is injured with a multiphoton laser
Dr. Ram Mohan - Organic Synthesis/ Green Chemistry
Please visit Dr. Mohan's Research Page to learn more about his research interests.
https://www.iwu.edu/chemistry/faculty/rmohan-research-interests.html
Dr. Manori Perera - Analytical and Astrochemistry
Perera’s lab has two unique areas of research.
First, astrochemistry, which is a relatively young field of research, addresses a gap in our understanding of molecular evolution in space. The current laboratory data are inadequate for identifying (1) most of the molecules and (2) many of the molecular processes at work in the interstellar medium (ISM). For instance, we do not yet understand the chemical processes associated with the creation and evolution of even the most basic molecules such as water and methanol in space. An important step toward understanding the chemistry of the ISM is to identify, through laboratory and theoretical work, a list of molecules that are present in the ISM. Perera group’s research focuses on contributing to this list through studies of ion-neutral reactions that are likely to be the dominant reaction processes in the cold cores of interstellar clouds. For this purpose, Perera group is building a specialized ion beam instrument at IWU with an ion trap and time-of-flight mass spectrometer. When the instrument is built we will be able study reaction dynamics of various ion-neutral reactions and contribute to astrochemistry by identifying molecules that may exist in the ISM and by providing an understanding of the reaction mechanisms relevant to these molecules.
Second, studies related to antioxidant in plants. Polyphenols in plants can offer many health benefits. These polyphenols are called antioxidants by consumers and theses particular types of molecules are advertised by the tea and wine industry in many of their commercials. In our lab, we studied how fermentation of tea affects the antioxidant content and reactivity. In addition, various additives (fruits or spices) are added to enhance the taste of black and green tea. Studies have shown that addition of milk results in decrease in antioxidants. Therefore, we are exploring the effects on tea due to various additions. We are extending this study of teas into another plant-derived product, wine. It is known that grapes contain similar antioxidants to tea and a few others that differentiate from tea. We would like to identify these antioxidants, quantify and measure their reactivity, based on the type of wine. The type of wine will range from white to red, as well as wine coming from different types of grapes.
Manori Perera Publications:
Nathan Hocker, Chaoqiuyu Wang, Jennifer Prochotsky, Atul Eppurath, Lydia Rudd, and Manori Perera, “Quantification of Antioxidant Properties in Popular Leaf and Bottled Tea by High-Performance Liquid Chromatography (HPLC), Spectrophotometry, and Voltammetry,” Analytical Letters, 2017, 50, 10, 1640-1656
Manori Perera, “A Colorful Quantification of Antioxidants in Tea: A First-year Undergraduate Laboratory Experiment,” Chemical Educator, 2016, 21, 273-277
Manori Perera, Kevin M. Roenitz, Ricardo B. Metz, Oleg Kostko and Musahid Ahmed, "VUV Photoionization Measurements and Electronic Structure Calculations of the Ionization Energies of Gas-Phase Tantalum Oxides TaOx (x=3-6)," Journal of Spectroscopy and Dynamics., 2014, 4, Art. No. 21 (10 pgs.)
Dr. Timothy Rettich - Physical Chemistry
The interactions of gaseous compounds with water have wide ranging applications in thermodynamics and kinetics. Highly precise measurements of the solubility of gases in water at varying temperatures provide a route for the determination of the enthalpy, entropy, and heat capacity changes for solution. These data in turn provide necessary tools for modeling the changes in the structure of water upon incorporation of small mole fractions of solutes. When the dissolved gas includes NOx, the solutions formed also react with near ultraviolet radiation to form hydroxyl and other radicals. When organic scavengers are added to the aqueous solutions formed from NOx, one has a robust model system for aerosols and natural ground water in a polluted environment. Rate constants and quantum yields determined from these model solutions play a key role in better understanding the more complex real world systems.
Timothy Rettich Publications:
Rettich, T. R.; Battino, R.; Wilhelm, E. High-Precision Determination of Henry Fugacities for Oxygen in Liquid Water from T = 274 K to T = 328 K J. Chem. Thermodyn. 2000, 32, 1145-1156.
Tominaga, T; Park, T.; Rettich, T. R.; Battino, R. Binary Gaseous Diffusion Coefficients. 7. Tetrachloroethene and 1,1,1-Trichloroethane with Methane and Tetraflouromethane at 100 kPa and 283-343 K. J. Chem. Eng. Data 1988, 33, 479-481.
Rettich, T. R.; Battino, R.; Wilhelm, E. Solubility of Gases in Liquids. XVI. Henry's Law Coefficients for Nitrogen in Water at 5 to 50 ˚C. J. Solution Chem. 1984, 13, 335-348.
Dr. Rebecca Roesner - Inorganic Chemistry
Polyoxometalates (POMs) are large, early-transition-metal oxide clusters that are widely studied owing to their catalytic and biological activity. But, because of their large size, POMs were historically outside the scope of anion recognition chemistry. Recently, however, there has been growing interest in the interactions between POMs and various organic molecules and solid supports. The Roesner group designs macrocyclic and polymacrocyclic receptors as hosts for POM guests. In these receptors, metallated crown ether moieties or protonated aza-macrocycles serve as pre-organized points of positive charge for coulombic interaction with the POM guests. The Roesner group is also interested in using the POM forms of anion exchange resins as oxidation catalysts and in attaching macrocyclic POM receptors to solid supports. In addition to their work with POMs and macrocycles, Dr. Roesner's group works with Dr. Thushara Perera (IWU Physics) to prepare mineral dusts for astrochemically relevant IR studies and has developed multi-week student laboratory projects on topics including ferrocene and biodiesel fuel.
Rebecca Roesner Publications:
Korendovych, I. V.; Roesner, R. A.; Rybak-Akimova, E. V. Molecular Recognition of Neutral and Charged Guests Using Metallomacrocyclic Hosts. In Advances in Inorganic Chemistry; van Eldick, R.; Bowman-James, K. Eds.; Academic Press, 2006; Vol. 59, pp. 109-173.
Roesner, R. A.; McGrath, S. C.; Brockman, J. T.; Moll, J. D.; West, D. X.; Swearingen, J. K.; Castineiras, A. Mono- and di-Functional Aromatic Amines with p-Alkoxy Substituents as Novel Arylimido Ligands for the Hexamolybdate Ion. Inorg. Chim. Acta, 2003, 342, 37-47.
Roberts, K. M.; Cotner, S. E.; Schultz, T. C.; Roesner, R. A.; House, J. E. The Solubility of Ferrocene: An Experiment involving Synthesis, Instrumental Analysis, and Physical Properties. The Chemical Educator 2013, 18, 18-20.
Dr. Wathsala Waduge
The main objective of my research is exploring new chemistries to deposit inorganic thin films. Volatile and reactive organometallic complexes will be synthesized, characterized and evaluated for thermal properties to be used as precursors for thin film depositions.
Education
Ph.D. in Chemistry (Inorganic Chemistry), Wayne State University, February 2019.
B.S. (Special Degree) in Chemistry (First Class Honors), University of Ruhuna, Sri
Lanka, 2010.
Wathsala Waduge Publications
Solid-Phase Epitaxy of Perovskite High Dielectric PrAlO3 Films Grown by Atomic Layer
Deposition for Use in Two-Dimensional Electronics and Memory Devices,” Wathsala L.
I. Waduge, Y. Chen, P. Zuo, N. Jayakodiarachchi, T. F. Kuech, S. E. Babcock, P. G.
Evans, and C. H. Winter, ACS Appl. Nano Mater. 2019, 2, 7449-7458

Ram Mohan - Wendell and Loretta Hess Endowed Professor of Chemistry and Chair of Chemistry
Department - Chemistry